Spotlight Archive
February 2011
The mechanism by which hair cells are believed to sense the miniscule stimuli experienced in the ear is sketched in Fig. 4.8 in the text. Arrayed stereocilia exert forces on one another, opening activating ion channels; the signal sent by the hair cell is proportional to the number of open channels. Lim and Park recently modeled such a system, in which each member of a row of 31 cilia, modeled as inverted pendulums hinged at their base, was connected to its neighbors by a mechanical element with a spring constant that mimicked the gating spring. The channel gates were modeled by bistable elements, so the channel opened instantly once the opening force reached a threshold value. The authors also constructed a physical model consisting of five aluminum rods, which showed behavior similar to that predicted by the numerical model. Both the experimental and numerical models confirmed the notion that the high sensitivity of the hair cell arises because the opening of the channel causes a nonuniform softening of the sensory bundle, which has the effect of amplifying the response.
Ref: Lim and Park, A mechanical model of the gating spring mechanism of stereocilia, J Biomech 42:2158-2164, 2009.May 2010
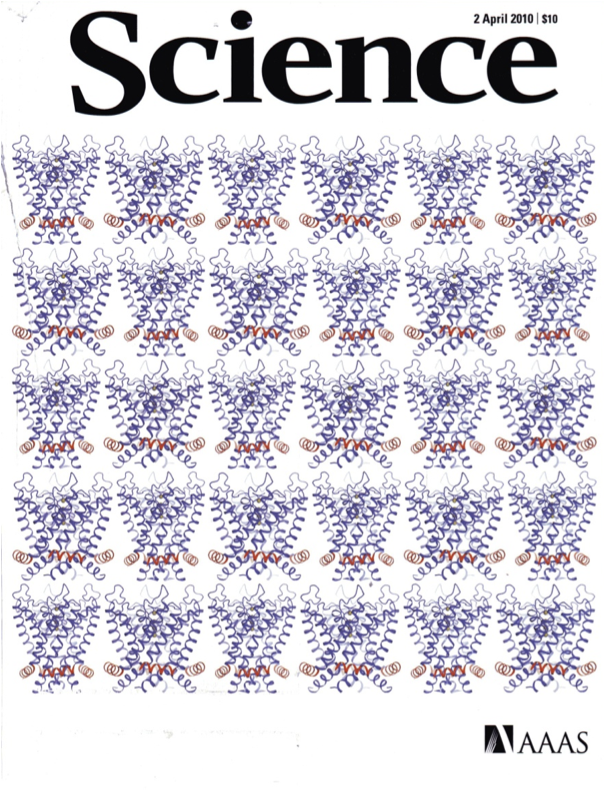
Figure 4.6b in the text contains two images of a portion of a voltage-dependent potassium channel (the Kv1.2 channel in rat brain), showing the conformational change of a region of the protein thought to serve as the voltage sensor; the figure is reproduced in color facing p. 382 in the text. Fifteen replicates of this figure grace the cover of the 2 April 2010 issue of Science, owing to further work by the MacKinnon lab, reported in that issue, in which they identify and locate the amino acids on the S4 segment that are responsible for the movement of the segment and consequent gating of the channel. They represent the gating process through a state diagram (e.g., Sections 4.2.2 and 9.1.3) in which each of the four S4 segments (one on each subunit), independent of one another, passes through four serial transitions from closed to open, and the channel opens only when all four segments are in the open state.
Ref: Tao, Lee, Limapichat, Dougherty and MacKinnon, A gating charge transfer center in voltage sensors, Science 328:67-73, 2010.
February 2010
We saw in Chapter 9 that the flow of water across the capillary wall involves passage through the negatively charged glycocalyx (Chapter 3) on the luminal side and then through intercellular clefts to the external interstitium. Smaller solutes in the plasma also cross these two layers by convection and diffusion. When the solutes are charged, their concentration in the glycocalyx is modified through a Donnan effect (Chapter 1); negatively charged species are disproportionately excluded from the barrier and their flux is accordingly reduced. Chen and Fu modeled the convective diffusion of charged solutes through the glycocalyx and cleft, including electrostatic potential gradients and solute partition at the boundaries of the glycocalyx, as in Fig 2.7. They showed that the reduced interstitial concentration of labeled serum albumin, each molecule of which carries 19 net negative charges, relative to that of an uncharged dextran of the same size, could be explained solely as a result of exclusion of the former from the glycocalyx.
Ref: Chen and Fu, A time-dependent electrodiffusion-convection model for charged macromolecule transport across the microvessel wall and in the interstitial space, Cell Mol Bioeng 2:514-532, 2009 .
November 2009
The recycling of neurotransmitter vesicles shown in Fig. 9.19 is called “kiss-and-run” when it takes place without full vesicle collapse into the presynaptic membrane. Kiss-and-run recycling is only one of several alternative recycling processes that can take place after transmitter release. In “full-collapse fusion”, the emptied vesicular membrane collapses into the presynaptic membrane and loses its identity after release, so it cannot be reused. The synthetic demand on the neuron is increased as more vesicles undergo full-collapse fusion and fewer kiss-and-run. Zhang et al. distinguished between these two processes by loading individual vesicles with quantum dots (Qdots) 15 nm in diameter, too large to pass through the fusion pore (Chapter 3) that forms during exocytosis. Thus vesicles undergoing kiss-and-run would retain their Qdots upon transmitter release, while those undergoing full-collapse fusion would lose theirs. The opening of the vesicle could also be detected, because the photoluminescence of the Qdots was pH-sensitive, and the intravesicular fluid is considerably more acidic than the synaptic cleft fluid, which enters the vesicle through the fusion pore during transmitter discharge. The authors found that kiss-and-run recycling dominated when nerve activity was high and the synthetic capability of the cell was stressed, and full-collapse fusion was more common at lower stimulation rates.
Ref: Zhang, Li and Tsien, The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles, Science 323:1448-1453, 2009.
August 2009
A recent issue of Microcirculation is devoted to a series of articles on the topic "Theoretical Modeling of the Microcirculation". The opening article of the issue by Secomb et al describes the variety of models that are used to describe microcirculatory processes, and biological transport processes in general. Four model types are described: phenomenological models, in which empirical equations are used to fit experimental data; qualitative conceptual models, in which a hypothesis is translated into equations and is evaluated by the ability of the equations to describe the behavior of experimental data in a qualitative sense; quantitative conceptual models, which are similar to the preceding, except that the parameters of the equations are adjusted as in the phenomenological model and the validity of the model is tested by the quality of the fit and the plausibility of the best-fit parameters; and predictive models, in which the processes and parameters are so well understood that the model can be used to predict the behavior of the system being modeled. Examples of all four types of model are given.
Among the papers in the issue is a review by Goldman of theoretical models of microvascular oxygen transport, that parallels the discussion in Chapter 11; it also includes a summary of multispecies models that describe the interaction between oxygen transport and that of CO2, NO and glucose.
Refs: Secomb, Beard, Frisbee, Smith and Pries, The role of theoretical modeling in microcirculation research, Microcirculation 15:693-698, 2008; Goldman, Theoretical models of microvascular oxygen transport to tissue, Microcirculation 15:795-811, 2008.
May 2009
Secondary active transport relies on carrier proteins that undergo conformational change when bound to cosolute and substrate. Two modes of conformational change are the "alternating access" (or "rocker switch") mode, in which the accessibility to the substrate binding site alternates between the two sides of the membrane, and a gated channel-like (or "two-gate") mode, in which gates at the two ends of a substrate-binding core compartment alternately open and close (see Fig. 4.11). The rocker switch mode has been demonstrated for several carriers. Faham et al. recently demonstrated for the first time a transporter that uses the two-gate mode, the Na-galactose symporter of the bacterium, /V. parahaemolyticus/, This symporter is similar to SGLT-1, which is responsible for glucose uptake in the intestine. Using x-ray diffraction at 0.27 nm resolution, they showed that the protein assembles as an anti-parallel dimer. Each half of the dimer has a hydrophobic gating segment positioned at a membrane face, so the two in combination generate the two-gate topology. In the protein conformation examined by Faham et al, both gates were closed, yielding the occluded state which is a distinguishing feature of the two-gate model.
Ref: Faham, Watanabe, Besserer, Cascio, Specht, Hirayama, Wright and Abramson, The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport, Science 321:810-814, 2008
February 2009
The stomach is known to exhibit a rhythmic electrical activity known as the gastric slow wave, whose origin is still uncertain. Corrias and Buist use mathematical modeling of the pacemaker cells that generate the wave to suggest a mechanism. Their model includes the cell membrane and various resident channels, the endoplasmic reticulum (ER), mitochondria, and a small submembrane space (SS) that functions as a separate compartment. The description of transmembrane transport is reminiscent of the Hodgkin-Huxley model of axonal conduction: currents are described by equivalent circuits with voltage-dependent gating variables, and the capacitance of the cell membrane is included in the unsteady solution. The process is initiated by calcium release from the ER into the SS. Calcium in the SS opens a Ca transporter in the mitochondrial membrane. The ion flows into the mitochondrion, reducing its level in the SS, and allowing a Ca-inhibited cation channel in the cell membrane to open. This depolarizes the cell, initiating the slow wave. The model successfully describes the frequency, amplitude and shape of the wave.
Ref: Corrias and Buist, Quantitative cellular description of gastric slow wave activity, Am J Physiol Gastrointest Liver Physiol 294:G989-G995, 2008
May 2008
The mechanism by which the corneal endothelium pumps water from the corneal stroma to the aqueous has been of interest for many years. Diecke et al present results using rabbit corneas that suggest that perhaps 30% of the normal water flux continues in the absence of any concurrent net solute transport. They propose that, in this state, there are equal inward-directed transcellular and outward-directed paracellular fluxes of sodium, and that the latter flux drives an electroosmotic flow of fluid into the aqueous.
It is interesting to compare this result with the description of flow through a parallel path membrane in Chap. 10, where recirculation of volume flow causes an increment in solute flux. Both phenomena are due to the coupling of a flux to a non-conjugate force: electroosmosis in Diecke et al, and convection in Chap. 10.
Ref: Diecke, Ma, Iserovich and Fischbarg, Corneal endothelium transports fluid in the absence of solute transport, Biochim Biophys Acta 1768:2043-2048, 2007