SPOTLIGHT
February 2011
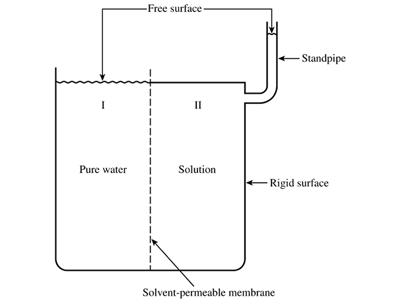
In Chapter 6, we introduce the notion of osmotic pressure, which is a driving force for solvent flow that arises from differences in the composition of the solutions on the two sides of a semipermeable membrane. On p. 240, we note that substantial osmotic pressures can be generated by relatively small differences in total concentration; 1 mM can generate 19 torr of pressure at 37C. Recently, a Norwegian power company, Statkraft, has developed a plant that uses osmotic pressure to generate electricity: water flowing across a membrane from a freshwater chamber into a seawater chamber runs through a turbine, generating 4 kW from 2000 square meters of membrane. It is easy to see how this might work using the osmometer sketch in Fig. 6.4. Phase II is fresh water and Phase I is sea water. Suppose the height of the tube were less than h, the height at which the hydrostatic head balances the osmotic pressure difference across the membrane. Then the head could never get high enough to stop the flow, and if the salinity of Phase I were maintained, salt water would continuously overflow from the top of the tube, and could be channeled as it flowed downward to power a turbine.
Refs: Winters, Osmosis Power, Mechanical Engineering 132:10, 2010.
http://www.statkraft.com/energy-sources/osmotic-power/osmotic-power-in-brief/
Spotlight Archive